Specialized technology:
Our strong chemistry: Friedel Crafts reaction, Bromination reaction, Chlorination reaction, Grignard reaction, Coupling reaction, Chiral asymmetric synthesis(including asymmetric reduction, alkylation), Heterocyclic compounds synthesis, Reduction reaction, Hockman re-arrangement reaction, as well as other basic regular reactions.
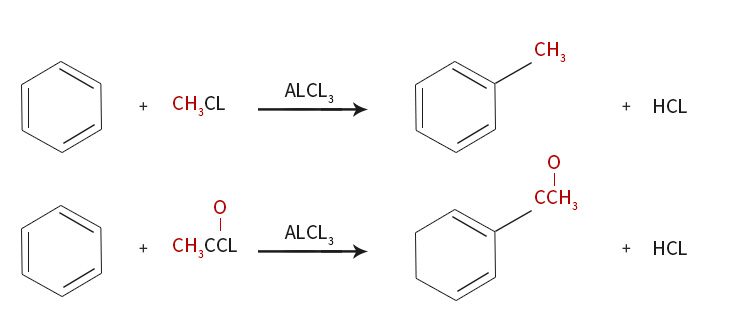
Friedel-Crafts reaction
Friedel Crafts is classical synthesis method of acylation processes.Some examples:Ar─H+R-COCl→Ar-CO-R+HCl
Grignard reaction
Grignard reaction is classical synthesis method of alkylation's processes. We routinely prepares and makes use of Grignard reagents for the manufacture of intermediates and active ingredients for the markets of Pharmaceuticals and chemicals. Syntheses are performed at scales between some tens of kg and more than 500kg per batch because of local dry air condition.
R-X+Mg→R-MgX
RMgX + H2O → RH + Mg(OH)X
RMgX + ROH → RH + ROMgX
RMgX + RCOOH → RH + RCOOMgX
RMgX + NH3 → RH + NH2MgX
RMgX + RNH2 → RH + RNHMgX
RMgX + RC≡CH → RH + RC≡CMgX
Bromination reaction
Bromination reaction is classical synthesis method of Bromide series.
CH3COOC2H5 + Br2 → BrCH2COOC2H5 + HBr
ClCH2C≡CH + HBr → ClCH2CH2CH2Br
3 RCOOH + PBr3 → 3 RCOBr + HP(O)(OH)2
Acyl chlorination
Sulfuryl dichloride, oxalyl dichloride, chlorine gas and chlorine hydride are often used as chloride agent. Some examples :
R-COOH + SOCl2 → R-COCl + SO2 + HCl
R-COOH + PCl3 → R-COCl + HOPCl2
Nitration
Organic compounds in molecules into nitro (-NO2) reaction. Aromatics of nitration is the preparation of aromatic nitro-compound the most important methods. Nitration reagent have nitric acid and denitration acyl bittern (NOX), four fluorine boric acid nitro cation, etc, and most important, the most commonly used is the mixture of sulfuric acid and nitric acid.
Ar─H+HNO3→Ar─NO2+H2O
C7H8 + HNO3 → C7H7NO2 + H2O